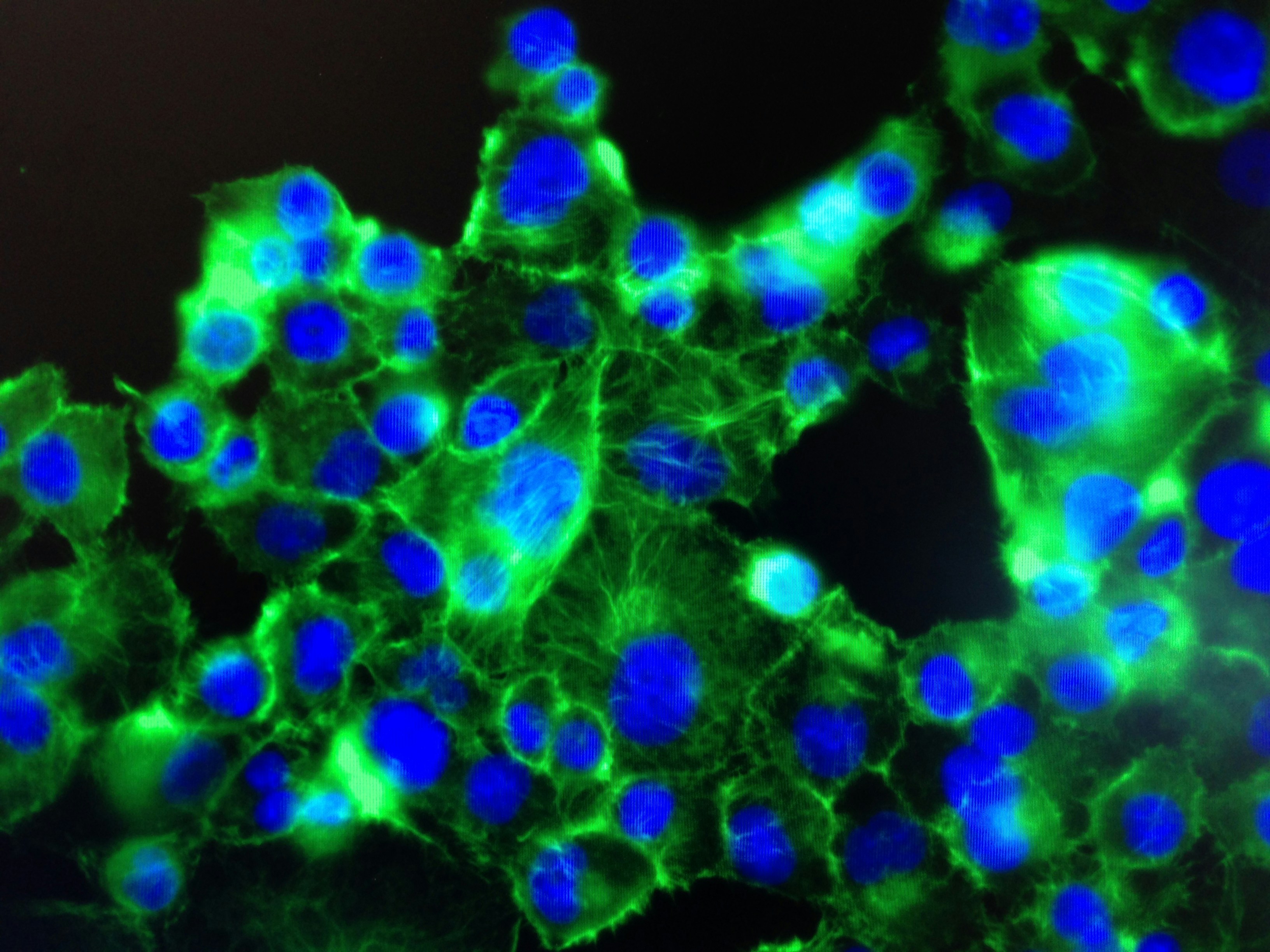
Introduction to Phenotype and Genotype
In biology, the concepts of phenotype and genotype are foundational to the understanding of heredity and the expression of traits within living organisms. The genotype of an organism encompasses its complete set of genes and indicates the specific alleles present. This genetic makeup plays a crucial role in determining various characteristics, as it provides the blueprint for development and functioning. For example, the genotype may specify genes related to eye color, height, or susceptibility to certain diseases.
In contrast, phenotype refers to the observable characteristics or traits of an organism that result from the interaction between the genotype and environmental factors. These traits can include physical attributes, such as color and shape, as well as behavioral aspects. Phenotypes are not solely dictated by an organism’s genetic composition but are also influenced by environmental variables, such as nutrition, climate, and lifestyle choices. This means that two organisms with the same genotype may exhibit different phenotypes if they are exposed to different environments.
The significance of understanding phenotype and genotype extends beyond basic biology; it impacts fields such as medicine, agriculture, and conservation. In cancer research, for instance, identifying changes in genotype can lead to insights into tumor development and progression. Similarly, studying phenotypic variations helps researchers understand how cancer manifests differently among individuals. By examining both genotype and phenotype, scientists can gain a comprehensive view of biological diversity, evolutionary processes, and the complex interplay of genetics and environment, which is essential for advancements in personalized medicine and targeted therapies.
The Impact of Environment on Phenotype
The phenotype of an organism is not solely determined by its genotype; rather, it is significantly influenced by its environment. Various environmental factors such as temperature, nutrition, and exposure to toxins can profoundly alter the expression of phenotypic traits. This interplay between genotype and environmental conditions is a key area of interest in cancer research, as understanding how these factors affect cellular behavior can provide insights into disease progression and treatment options.
One notable example of the influence of environmental conditions on phenotype is phenotypic plasticity. This phenomenon occurs when organisms exhibit different traits in response to varying environmental stimuli. For instance, certain plants can alter their size, shape, and leaf characteristics depending on the availability of sunlight and nutrients. Similarly, some animals can change their coloration or reproductive strategies in response to climate changes or habitat modifications.
Furthermore, nutritional availability plays a crucial role in shaping phenotypic outcomes. An organism’s access to essential nutrients can lead to variations in growth rates, reproductive success, and even susceptibility to diseases, including cancer. For example, deficiencies in specific nutrients have been linked to increased cancer risk, illustrating the connection between dietary habits and phenotypic outcomes that could contribute to disease pathology.
Additionally, exposure to environmental toxins, such as heavy metals, pesticides, and pollutants, can also induce phenotypic changes, which may promote the development of cancer. These environmental influences underscore the necessity of incorporating ecological and environmental factors when studying genetics and phenotypes, as they can provide a more holistic understanding of how traits are expressed in various contexts.
Differences Between Cancer Cells and Normal Cells
Cancer cells exhibit several distinguishing features that set them apart from normal cells, fundamentally altering their behavior within the body. One of the most notable characteristics is their tendency for uncontrolled growth. While normal cells adhere to regulatory mechanisms that govern their division and lifespan, cancer cells bypass these controls, leading to unregulated proliferation. This uncontrolled growth allows tumors to form, which can invade neighboring tissues and organs, emphasizing the aggressive nature of cancer biology.
Another key difference is the lack of differentiation in cancer cells. Normal cells typically have specific functions and characteristics that define their role within a tissue type. In contrast, cancer cells often become undifferentiated, losing the specialized functions that are present in their normal counterparts. This lack of differentiation contributes to the malignant nature of tumors, as cancer cells can become more aggressive and less responsive to traditional treatment methods. Moreover, within a tumor, a heterogeneity of cell types can arise, further complicating cancer treatment.
Genetic mutations also play a significant role in differentiating cancer cells from normal cells. Cancer cells frequently harbor multiple genetic alterations, which may include mutations in oncogenes or tumor suppressor genes. These mutations can drive the transformation of a normal cell into a cancerous one by promoting unchecked cellular growth or preventing apoptosis, the programmed cell death that typically eliminates defective cells. Understanding these genetic changes is crucial for developing targeted therapies and interventions in cancer treatment.
These differences between cancer cells and normal cells not only underscore the complexities of cancer biology but also highlight the importance of ongoing research in identifying new therapeutic targets. By comprehending these fundamental distinctions, researchers can enhance their approaches to treatment and improve outcomes for patients affected by cancer.
Studying Cancer Cell Phenotypes
Analyzing cancer cell phenotypes is essential for understanding their distinct behaviors and characteristics compared to normal cells. Various techniques have been developed to study these phenotypic differences effectively. Among the most prominent methods are microscopy, flow cytometry, and gene expression profiling.
Microscopy remains a cornerstone in the analysis of cell phenotypes. Techniques such as fluorescence microscopy allow researchers to visualize specific cellular components, enhancing our understanding of the structural variations present in cancer cells. By employing various staining protocols, researchers can identify abnormal morphologies or cellular features, such as increased size or irregular shapes that are often associated with malignancies.
Flow cytometry is another powerful tool utilized to study cancer cell phenotypes. This technique enables the simultaneous measurement of multiple physical and chemical characteristics of individual cells as they flow in a fluid stream through a laser. By analyzing the expressed surface markers or internal proteins, flow cytometry can assist in the identification of distinct cancer cell populations, providing insights into their physiology and response to treatment.
Gene expression profiling, particularly through techniques such as RNA sequencing, provides a deep understanding of the molecular alterations that underpin cancer cell behavior. By comparing the gene expression patterns of cancerous cells to those of normal cells, researchers can identify specific oncogenes and tumor suppressor genes that contribute to the malignancy. This profiling is critical for uncovering the underlying mechanisms of tumor progression and can help guide personalized treatment approaches.
In conclusion, through methods such as microscopy, flow cytometry, and gene expression profiling, researchers can elucidate the distinct phenotypic traits of cancer cells. These insights not only enhance our understanding of cancer biology but also contribute significantly to the development of targeted therapies.
Determining Cancer Cell Characteristics
Assessing whether cells are cancerous involves a range of techniques that enable researchers to evaluate cellular characteristics systematically. One of the most common methods used is histopathology, where tissue samples are examined microscopically for morphological alterations. This technique allows pathologists to identify abnormal cell growth, an essential hallmark of cancer, as well as to classify tumor types based on histological features.
Molecular profiling is another significant approach in determining cancer cell characteristics. This method utilizes advanced techniques like next-generation sequencing (NGS) and RNA sequencing to analyze the genetic and epigenetic alterations present in cancer cells. By identifying mutations, copy number variations, and expression levels of genes, researchers can paint a comprehensive picture of the tumor’s biological behavior. Molecular profiling also plays a crucial role in personalized medicine, where treatments can be tailored according to the specific genetic profile of an individual’s cancer.
Additionally, various assays for detecting specific biomarkers associated with cancer are increasingly employed in both research and clinical settings. These biomarkers may include proteins, DNA, or RNA that signify the presence of malignant cells. Tests such as immunohistochemistry (IHC) enable visualization of specific antigens in tissue samples, providing valuable insights regarding tumor cell type and prognosis. Moreover, liquid biopsies, which analyze tumor-derived materials from bodily fluids, offer a non-invasive approach for assessing cancer presence and treatment response.
The identification of these biomarkers is paramount in cancer diagnosis and treatment and aids in the stratification of patients into appropriate therapy regimens. Accurate determination of the cancer cell characteristics not only enhances the understanding of cancer biology but also improves patient outcomes through informed clinical decisions.
Gene Expression in Cancer vs. Normal Cells
Gene expression plays a pivotal role in the distinction between cancerous and non-cancerous cells, particularly under various stress conditions. In normal cells, gene expression is typically regulated, ensuring a balance that maintains cellular functions and homeostasis. However, when cells are exposed to stressors such as hypoxia and oxidative stress, significant alterations in the gene expression profiles can occur, which may contribute to tumorigenesis. Hypoxia, characterized by low oxygen levels, is a common physiological condition in solid tumors. Under these circumstances, cancer cells often activate specific pathways that promote angiogenesis, cell survival, and metastasis, all of which are essential for tumor progression.
Research has demonstrated that oxidative stress, which results from an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify them, can also lead to abnormal gene expression. Cancer cells frequently exhibit altered antioxidant response pathways, allowing them to survive and thrive in environments that would be detrimental to normal cells. This inherent capacity to adapt gene expression under stress not only fuels the malignancy but also presents challenges for treatment strategies, as these pathways can lead to resistance against therapies.
Advancements in technologies, such as RNA sequencing and quantitative polymerase chain reaction (qPCR), have revolutionized our understanding of gene expression in cancer research. These techniques allow for high-throughput analysis of gene expression profiles, enabling researchers to identify specific genes that are overexpressed or repressed in cancer cells compared to normal counterparts. Such insights are crucial for developing targeted therapies, understanding tumor biology, and improving patient outcomes.
Studying the differential gene expression under conditions of stress thus provides invaluable information on how cancer cells adapt and evolve, highlighting the need for continued research in this critical area of cancer biology.
The Role of Genetic Mutations in Cancer Phenotype
Genetic mutations play a crucial role in the manifestation of cancer phenotypes, leading to altered cellular characteristics that define cancerous cells. These mutations can significantly impact gene function and regulation, contributing to the uncontrolled growth and proliferation that characterize cancer. Generally, genetic mutations fall into several categories, including point mutations, insertions, and deletions, each having distinct implications for gene expression and protein function.
Point mutations are the most common type of genetic alteration in cancer. They involve the substitution of a single nucleotide, potentially leading to changes in amino acid sequences within proteins. This can result in the production of proteins that are either hyperactive or dysfunctional, ultimately contributing to the malignancy. For instance, a mutation within an oncogene may lead to an overactive protein that drives cell division, exemplifying how a single nucleotide change can have profound effects on cellular behavior.
Insertions and deletions, on the other hand, involve the addition or loss of sequences in the DNA strand, which may disrupt the reading frame of a gene. Such alterations can lead to frameshift mutations, resulting in entirely different and often non-functional proteins. This disruption can significantly affect pathways that regulate cell growth, apoptosis, and DNA repair mechanisms, further emphasizing the relationship between genetic mutations and cancer phenotype.
Connecting these genetic changes to phenotypic traits is vital in cancer research, as it allows scientists to understand how specific mutations drive the development and progression of tumors. Identifying the mutations that correlate with distinctive phenotypic features can also aid in the development of targeted therapies, making it imperative for researchers to continue exploring these genetic underpinnings in cancer biology.
Implications of Understanding Phenotype and Genotype in Cancer Treatment
The intricate relationship between phenotype and genotype holds significant implications for cancer treatment, particularly through the advancements in personalized medicine. By analyzing the genetic makeup of an individual, healthcare providers can tailor treatment plans that align with the unique characteristics of both the patient’s tumor and their broader genetic profile. This approach enhances therapeutic efficacy and helps minimize adverse effects by ensuring that treatments are specifically designed to target the cancer cells most effectively.
Targeted therapies have emerged as a cornerstone of modern oncology, owing much of their effectiveness to a thorough understanding of the underlying genotypes associated with specific cancer types. For instance, certain mutations in genes such as EGFR or BRAF are known to drive the proliferation of some tumor cells. By utilizing targeted treatments that inhibit the activity of these mutated genes, clinicians can more effectively shrink tumors or halt their progression. As a result, the shift towards therapies designed on genetic and phenotypic profiles can lead to improved patient outcomes and enhanced survival rates.
Moreover, the development of novel cancer therapies grounded in the analysis of patient-specific genetic data is a burgeoning area of research. Researchers are now exploring methods to combine multiple treatment modalities based on the interaction between a patient’s genotype and phenotype. Such innovative strategies could result in multi-faceted therapies that attack cancer from various angles, further improving the likelihood of successful treatment.
In summary, a comprehensive understanding of the interplay between phenotype and genotype in cancer research is not merely academic; it carries real-world implications that could revolutionize treatment protocols. As advancements in genetics continue to evolve, oncologists will increasingly be equipped to provide more personalized and effective cancer care.
Conclusion and Future Directions in Cancer Research
In the context of cancer research, understanding both phenotype and genotype is vital for developing effective diagnostics and therapies. The phenotype encompasses the observable characteristics of cancer cells, while the genotype offers insights into the underlying genetic makeup. Our exploration highlighted how these two aspects are interdependent; alterations in genotype can manifest in distinct phenotypes, influencing tumor behavior and patient outcomes.
The integration of genomic data with phenotypic analysis presents an exciting avenue for future research. Advances in technologies, such as next-generation sequencing and high-throughput imaging, allow researchers to gather extensive datasets that link genetic variations to specific phenotypic traits. This synergy can lead to a deeper understanding of cancer progression, heterogeneity, and treatment responsiveness.
Moreover, the continued study of the interactions between genotype and phenotype holds the promise of identifying novel biomarkers for early detection and targeted therapies. As we harness data from diverse populations, personalized approaches to cancer treatment can be tailored to individual genetic profiles and phenotypic characteristics, potentially improving clinical outcomes.
Future directions in cancer research must also prioritize a multi-disciplinary approach, incorporating insights from genomics, proteomics, and systems biology. By fostering collaboration between oncologists, geneticists, and data scientists, the field can unlock new strategies for combating cancer. Encouragingly, as understanding deepens, there is potential for breakthroughs in prevention, early intervention, and the design of new therapeutic agents directed towards specific targets.
As we look to the horizon of cancer research, it remains clear that the intricate relationship between genotype and phenotype will continue to be at the forefront, guiding the development of innovative solutions to one of humanity’s most challenging health concerns.